Aktionärs Wert glaube ich
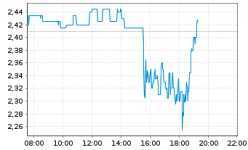
Nachbörslich News. Dänemark schließt um 17 Uhr....
Zealand Pharma announces positive topline results from 28-week Phase 1b trial with GLP-1/GLP-2 receptor dual agonist dapiglutide
June 18, 2025 11:29 ET
Company announcement – No. 15 / 2025
- Body weight reductions of a mean of 11.6% after 28 weeks with dapiglutide escalated up to 26 mg, with 95% of trial participants being male, a median baseline BMI of 28.5 kg/m2, and no lifestyle modifications
- Treatment with dapiglutide was assessed to be safe and well-tolerated with gastrointestinal adverse events consistent with the profile reported with other incretin-based therapies
- Unique, dual mechanism, including GLP-2 activity, designed to target obesity-related comorbid conditions driven by low-grade inflammation
Copenhagen, Denmark, June 18, 2025 – Zealand Pharma A/S (Nasdaq: ZEAL) (CVR-no. 20045078), a biotechnology company focused on the discovery and development of innovative peptide-based medicines, today announces positive topline clinical results from Part 2 of a Phase 1b multiple ascending dose (MAD) trial, investigating safety, tolerability, and clinical effects of 28 weeks of treatment with dapiglutide, a long-acting GLP-1/GLP-2 receptor dual agonist1.
“We are very encouraged by the impressive weight loss with dapiglutide after 28 weeks that appears on par with the most efficacious once-weekly GLP-1 receptor agonist-based therapy on the market today, despite the almost entirely male and relatively lean trial population,” said David Kendall, MD, Chief Medical Officer of Zealand Pharma. “Dapiglutide is a unique GLP-1 receptor agonist-based therapy, aiming to leverage a dual mechanism that includes GLP-2. Our mid- to late-stage obesity pipeline includes differentiated GLP-1 receptor agonist-based therapies that target people with obesity-related comorbid conditions, and our amylin analog, petrelintide, with potential as a foundational therapy for weight management, addressing unmet needs among the majority of people with overweight and obesity.”
In this Part 2 of the Phase 1b MAD trial, a total of 30 participants (~93% male) with a median age of 44.5 years, a median baseline body weight of 91.9 kg, and a median baseline BMI of 28.8 kg/m2 were randomized to receive 28 weekly doses of either dapiglutide or placebo (2:1) within one dose cohort. At week 28, the estimated mean body weight decreased by 11.6% from baseline among participants on dapiglutide treatment. Placebo treatment resulted in a mean body weight decrease of 0.2% from baseline2. No lifestyle modifications, such as diet or exercise, were included in the trial.
Higher doses of dapiglutide compared to the prior 13-week Part 1 of the trial were assessed to be safe and well-tolerated in the trial, with no severe or serious treatment-emergent adverse events (TEAEs) reported. The vast majority of TEAEs were mild and the most common were gastrointestinal (GI), including nausea and vomiting. Two participants withdrew due to TEAEs, one of which was related to GI events. Overall, the number of GI events observed was consistent with clinical trials of other incretin-based therapies. A low number of participants reported injection site reactions, all of which were mild.
About the Phase 1b trialThe Phase 1b trial is a single-center, randomized, double-blind, placebo-controlled clinical trial in participants with overweight or obesity (eligible BMI 27.0 to 39.9 kg/m2), investigating safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of dapiglutide as subcutaneous injections using a dose escalation scheme (NCT06000891). No lifestyle modifications, such as diet or exercise, are included in the trial. The trial consists of Part 1 and Part 2.
Part 1 included 54 participants in three cohorts receiving 13 once-weekly doses of dapiglutide or placebo, with rapid dose escalation every second week. Participants randomized to the highest dose cohort (13 mg) received the target dose for a period of 5 weeks. Topline results from Part 1 showed placebo-adjusted reductions in body weight of up to a mean of 8.3% with dapiglutide after 13 weeks (up to 6.2% mean weight loss on dapiglutide; 2.1% mean weight gain on placebo)3. Detailed results from Part 1 of the Phase 1b MAD trial with dapiglutide will be presented at the American Diabetes Association’s 85th Scientific Sessions in Chicago, Illinois on June 20, 2025.
Part 2 of the trial includes 30 participants receiving 28 once-weekly doses of dapiglutide up to 26 mg or placebo within one dose cohort, with dose escalation every fourth week.
https://www.globenewswire.com/news-...-GLP-2-receptor-dual-agonist-dapiglutide.html#R9B(A3EQD5)
2,29 -4,6%